New research suggests that tirzepatide, the active ingredient in Eli Lilly's popular weight loss drugs Mounjaro and Zepbound, may effectively reduce the severity of sleep apnea, a very common sleep disorder.
Published in the New England Journal of Medicine, the novel finding could reshape the clinical approach to sleep apnea and bolster the case for broader insurance coverage for GLP-1 receptor agonists, given their expanding range of medical benefits.
Exploring Tirzepatide's Potential Beyond Weight Loss
Tirzepatide has already gained attention for its efficacy in weight management and diabetes care. Now, it appears poised to address another major health concern: obstructive sleep apnea (OSA).
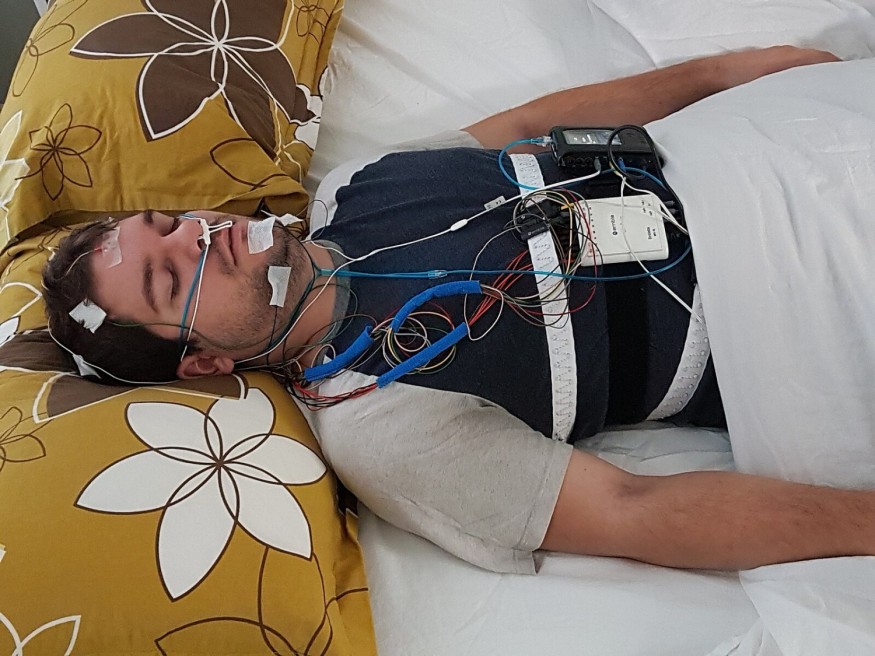
Characterized by repeated interruptions in breathing during sleep, OSA reportedly affects approximately 1 billion people worldwide and can lead to serious health conditions such as high blood pressure, heart disease, and diabetes.
Current treatments often involve continuous positive airway pressure (CPAP) machines or surgical interventions, which are not always well-tolerated by patients.
The latest study, which was a collaboration among researchers from nine countries and partly funded by Eli Lilly, involved 469 clinically obese individuals with moderate-to-severe OSA. Over a year, participants were administered varying doses of tirzepatide or a placebo.
The results were promising: tirzepatide significantly reduced the frequency of breathing interruptions during sleep, a key measure of OSA severity. Remarkably, some participants showed such improvement that they might no longer need CPAP therapy.
Dr. Atul Malhotra, the lead author of the study, noted the potential of tirzepatide to transform the treatment landscape for OSA.
"In the trials, patients with moderate-to-severe obstructive sleep apnea and obesity treated with tirzepatide experienced about 30 fewer disruptive events every hour of sleep and nearly half achieved disease resolution," the director of sleep medicine at UC San Diego Health said in a press release.
"OSA can be very disruptive to daily life and affects a person's long-term health when left untreated because it can lead to serious cardiometabolic complications. These data support the efficacy of tirzepatide in adults living with moderate-to-severe OSA and obesity and has the potential to add to our toolbox for OSA treatment."
Implications for Treatment and Insurance Coverage
Eli Lilly's findings come as the company prepares to submit data to the FDA and other global regulatory bodies to expand tirzepatide's approved uses to include sleep apnea treatment, according to Forbes.
The drug has already received fast-track designation from the FDA for this indication. If approved, tirzepatide would be the first pharmaceutical treatment targeting the underlying causes of OSA rather than merely alleviating symptoms.
This development could also influence insurance policies. Despite the high cost of GLP-1 drugs, their potential to treat a broader array of conditions may pressure insurers to reconsider coverage restrictions. Jeff Emmick, Lilly's senior vice president of product development, has pointed out the significance of addressing OSA, noting that many cases remain undiagnosed and untreated.
"There are currently no pharmaceutical treatment options to address the underlying cause of OSA, a complex disease that disrupts the daily lives of 80 million people in the U.S. alone and is linked to serious health complications," Emmick said.
Broader Benefits of GLP-1 Drugs
The findings add to a growing body of research supporting the benefits of GLP-1 receptor agonists like tirzepatide and semaglutide, which are also marketed for heart disease, kidney disease, and other conditions.
This expanding evidence base underscores that the drugs' benefits extend beyond weight loss and blood sugar management, suggesting other therapeutic mechanisms at work. Novo Nordisk, another major player in the GLP-1 market, has already seen success with its drug semaglutide, securing label expansion for its cardiovascular benefits.
